EXTRACTION AND SEPARATION OF NIOBIUM AND TANTALUM
The industrial separation of niobium and tantalum was achieved by applying fractional crystallization methods of potassium haptafluoromethalates (Marignac process) or by fluorotantalate by adding small amounts of MeiBuCO to the aqueous phase.
Finally, tantalum salt is extracted from an organic phase with water or diluted aqueous solution of ammonium fluoride. The yield of the process can exceed 95% and a tantalum salt with a purity of 99.9% can be achieved through sophisticated methods of optimization and process control.
Although other means of extraction have been cited in scientific literature, in the industry only the aforementioned methyl isobutyl ketone, tributyl phosphate (Bu3PO4) and tri-n-octylphosphine oxide [(n-C8H17) 3PO] have been used.
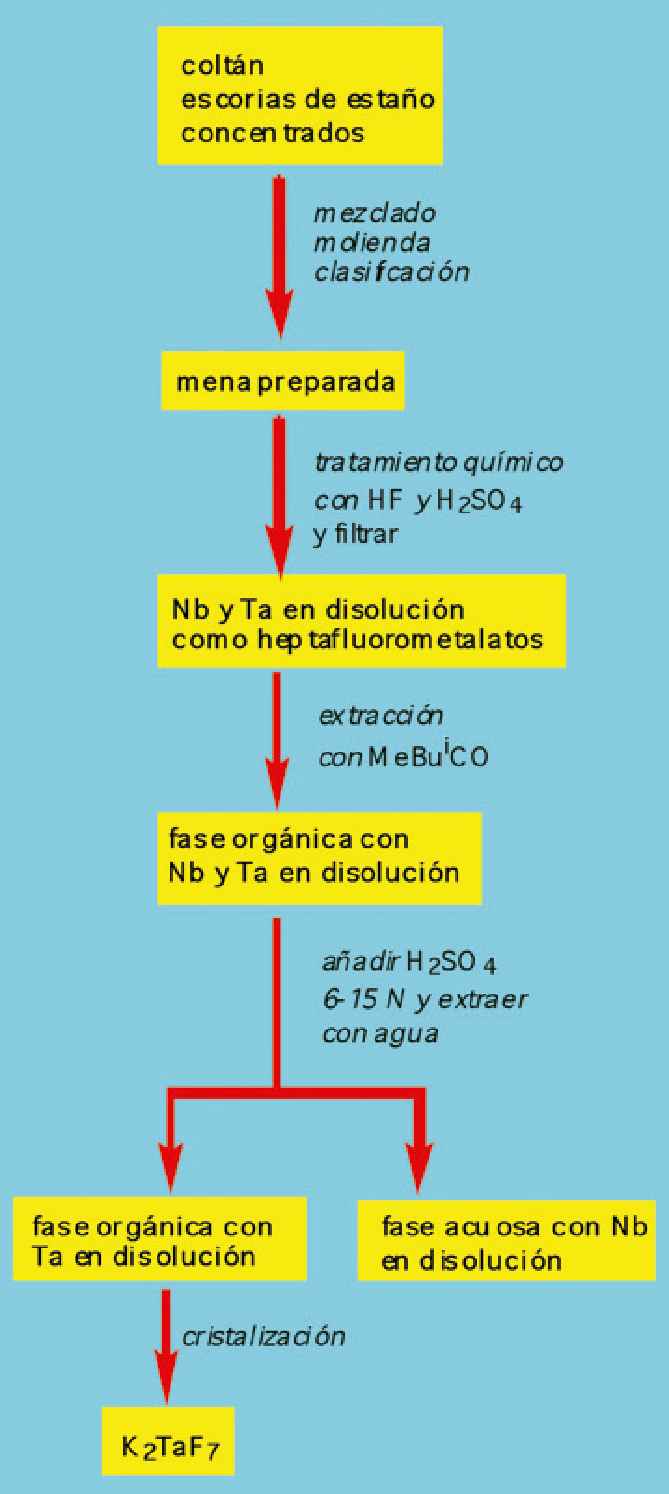
Figure 1. Extraction and separation of tantalum and niobium with methyl isobutyl ketone (MeiBuCO).
OBTAINING OF METAL TANTALUM
The only methods the preparation of metallic tantalum for commercial importance are the electrochemical reduction of tantalum oxide (Ta2O5) and chemical reduction of K2TaF7 with sodium.
However, tantalum produced by the first method did not have sufficient quality for its main application since the nineties, tantalum capacitors.
Almost all produced tantalum today is based on the reduction of K2TaF7 with metallic sodium.
On a commercial scale, the method was first used by Siemens & Halske AG, Berlin, at the beginning of the twentieth century.
Since then, due to high demand for high-quality powder tantalum for capacitors, the method has gradually been improved until reactor has been optimized as shown schematically in FIG. 3.
The reactor is usually 1 m in diameter and is covered with a lid provided with an agitator.
The reactor and its charge (K2TaF7 together with some diluent salt, such as KF) is introduced into an electric furnace and heated to a temperature sufficient to achieve the fusion of the salts (generally, below 1000 ° C), and, Then molten metal sodium is injected into the retort.
It is essential to fine control on the variables that affect the process (reduction temperature, type and concentration of diluent, speed of addition of sodium and efficiency of agitation), because the quality of the final product depends on them.
For example, a high reduction temperature favors the chemical quality of the tantalum, but decreases the surface area of the tantalum powder.
The reaction mass is cooled and extracted from the retort, crushed and leached, first with a dilute mineral acid and then with water, to separate the tantalum powder from other salts.
Subject to a drying and classification process, this primary metal powder is now ready to be processed and converted into sheets, rods, wires or quality powder for the condensers.
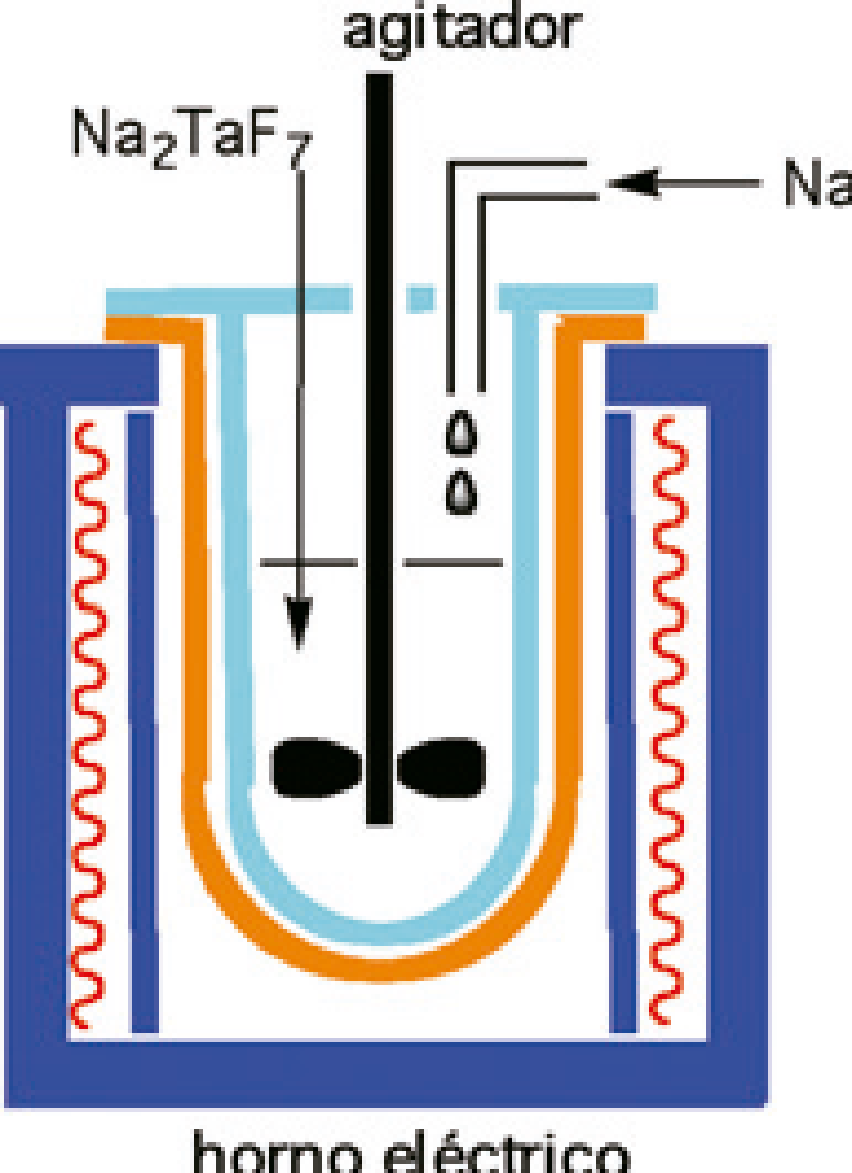
Figure 2. Schematic of the electric furnace used in the manufacture of metallic tantalum
APPLICATIONS OF TANTALUM
In Table 1 some physical properties of metallic tantalum are given.
It is a dense metal with a very high melting temperature (in fact, its first commercial use was as a filament in the incandescent lamps, although it was soon replaced by the tungsten) and a good conductor of electricity.
But its main applications derive from its excellent resistance to chemical corrosion, because it is coated with an adherent layer of oxide (Ta2O5) that acts as a protective layer on the metal surface.
Below 200 ° C it is not attacked by mineral acids or by water regia, and also inert to most organic compounds.
Only hydrofluoric acid or acid solutions containing fluoride can destroy the protective layer of the oxide with the consequent corrosion of the metal.
Table 1. Physical properties of metallic tantalum
-
Melting point / ºC 2.980,00 Boiling point / ºC 5.534,00 Enthalpy of fusion / kJ mol-1 24,70 Enthalpy of vaporization / kJ mol- 758,20 Atomization enthalpy / kJ mol- 782,00 Density (at 20ºC) / g cm-3 16,65 Electrical resistivity / ohm cm 12,40
For the above reasons, tantalum is used in the chemical industry for the manufacture of reaction vessels and heat exchanges in corrosive environments.
It is also used for medical purposes: in sutures, bone screws, cartilage wires, meshes for the reconstruction of the abdominal wall in hernia operations, dental implants, etc.
In these applications, in addition to the chemical inertness of the metal, its compatibility plays an important role.
In the nineties there was a spectacular increase in the demand for tantalum.
Almost 80 percent of the world production is oriented to the manufacture of solid tantalum capacitors for use in electronic devices.
TANTALUM CAPACITOR
In the manufacture of tantalum condensers, the tantalum powder is compressed into tablets of a few hundred milligrams, which are then sintered in vacuum at temperatures above 1300 ° C until a porous compact material is obtained.
The next step consists in the anodization of the tablet making it act as an anode in an electrolysis cell with a diluted aqueous solution of phosphoric acid as electrolyte.
It is forming an anodic tantalum oxide film on surface of the tantalum, whose thickness is proportional to the applied voltage.
This surface layer of oxide is an excellent electrical insulator and acts as a dielectric in the conductor-dielectric-conductor configuration typical of a capacitor.
One conductor is tantalum and the other is manganese dioxide (MnO2), a semiconductor that is deposited on the anodic surface of tantalum oxide by pyrolysis of manganese nitrate.
The anodic terminal is constructed with tantalum wire.
The assembly is encapsulated resulting in a miniaturized configuration as shown in Fig. 3 (or another variant thereof appropriate to the intended use).
Tantalum capacitors have a volumetric capacitance so high that they are ideal for use in miniaturized electronic systems such as mobile phones, manual video cameras or personal computers.
In addition, they are very stable to variations in temperature, which explains their use in environments as severe as the engine compartment of automobiles.
Their high reliability also determines that they are the preferred capacitors in such critical applications as in electronic components of aerospace devices, step markers or safety equipment.
Figure 3. Schematic representation of a tantalum chip-type capacitor.
CONCLUSION
The great industrial demand of miniaturized tantalum condensers has determined the resurgence of tantalum metallurgy and the setting of niobium-tantalum separation methods based on the extraction with organic solvents such as butylisobutyl ketone.
The reduction to metallic tantalum to state of dust of required quality in the tantalum condensers is achieved by reducing K2TaF7 with metallic sodium in an electric furnace, under specific conditions and with a very fine control of the variables that affect the process.
Tantalum capacitors are very stable to temperature variations and have great reliability even in harsh environments.
